Abstract
INTRODUCTION
Long-term survival in children with acute lymphoblastic leukemia (ALL) is achieved in around 80%; however, cure rates for adolescents and young adults (AYA) and older adults remain relatively low, at only 40% to 50%. The regimens used in adults are usually less intense than the ones used in their pediatric counterparts, mainly since adults do not tolerate the intensity as well as children do. Different studies have found that adolescents with ALL have a better outcome when treated with pediatric-inspired regimens compared to adult protocols, and other reports suggest improved outcomes for adult patients when treated with ALL pediatric-inspired combination chemotherapy (PICC) regimens. To this effect, we have designed a modification of the St. Jude's TOTAL XI combined chemotherapy program with the goal of delivering it on an outpatient basis. We report here the long-term results of a group of 126 non-pediatric patients with ALL treated with an asparaginase-containing PICC.
MATERIAL AND METHODS
All consecutive adolescents (12 - 17.9 years), young adults (18 - 24.9 years) and adult (>25 years) patients with ALL diagnosed and treated in the Centro de Hematología y Medicina Interna de Puebla since June 1983 were prospectively included in the study.
At diagnosis, the immunophenotype of the malignant cells and the desoxyrribonucleic acid (DNA) content was analyzed by flow cytometry, and cytogenetic markers were also obtained. Patients were treated with a modification of the St. Jude's TOTAL XI PICC regimen, delivered in an outpatient setting.
RESULTS
A total of 126 patients with ALL (69 AYAs and 57 adults were prospectively enrolled in the study. Median age was 22.5 years (range 12 - 80 years). B-cell lineage was identified in 117 (93%) and T-cell in 9 (7%). At diagnosis, 13 cases (11%) displayed more than 20 x 109/L white blood cells (WBC), 20 (16%) more than 50 x 109/L WBC, while 93 (74%) had less than 20 x 109/L WBC. DNA content was diploid in 69% of cases, hyperploid in 20% and hypodiploid in 11%. Twenty patients (21% of 94 tested) had a BCR::ABL1 rearrangement.
The median follow-up was 20 months (range 3 to 326 months). Of the 126 patients enrolled, 5 did not complete the first course of chemotherapy and were lost to follow up. Of the remaining 121 patients, early discontinuation occurred in 5 patients that had complications upon initial therapy and died 3 - 15 days after the diagnosis. Among the remaining 116 patients, 90 (77%) achieved a complete remission (CR) (71% on intention to treat analysis). Chemotherapy was initiated on an outpatient basis in all patients and 24 (20%) had to be admitted in the hospital during the initial 7-week period of the induction / consolidation.
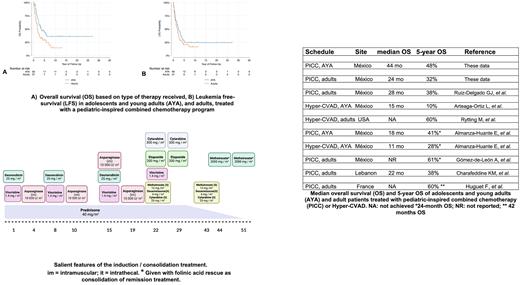
Relapses were observed in 38 of the 90 patients who achieved a CR (43%); 13 relapses presented in the CNS and 25 in the BM; two persons had both relapses. The median remission duration for the total cohort was 48 months, while the median overall survival (OS) for the total population was 30 months.
CONCLUSION
The use of an asparaginase-containing PICC in AYAs and adult patients with ALL renders acceptable results, which are better than those obtained in similar socioeconomic circumstances using adult-oriented treatments, such as Hyper-CVAD. The toxicity of this regimen is acceptable and since it can be delivered on an outpatient basis, it results in diminished costs.
Disclosures
Cortes:Bristol Myers Squibb: Consultancy, Research Funding; Kartos: Research Funding; Biopath Holdings: Consultancy, Current equity holder in private company; Abbvie: Consultancy, Research Funding; Forma Therapuetic: Consultancy; Novartis: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Research Funding; Sun Pharma: Consultancy, Research Funding; Gilead: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal